Which Word Describes the Energy in an Exergonic Reaction
There are several bonds broken and formed in Equilibrium 1 that you must account for. In a theoretical world where all things are possible how could you increase the amount of energy that could be.

Quia 9ap Chapter 8 An Introduction To Metabolism Basic
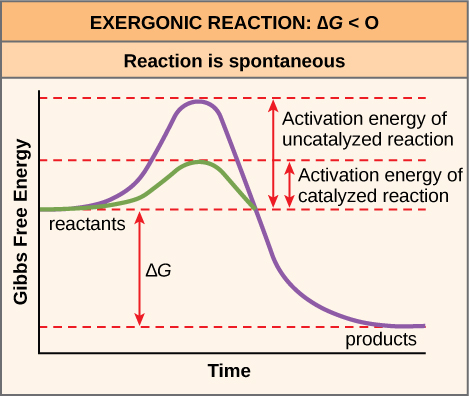
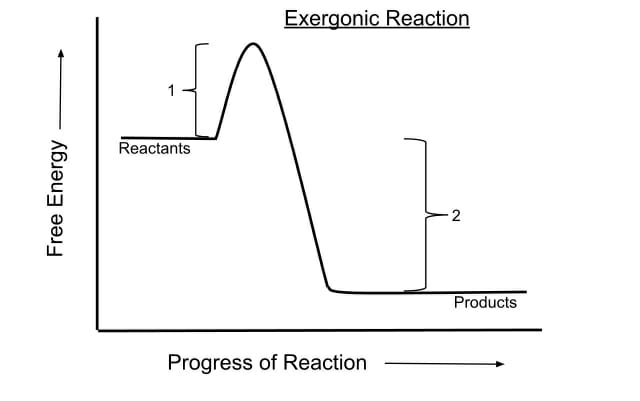
This kind of reaction is termed EXERGONIC or if it releases heat exothermic.

. Releases energy and is always spontaneous. This process repeats until deoxyribonucleotide triphosphates run out or the replication complex falls off of the DNA. This reaction releases pyrophosphate and couples the exergonic hydrolysis of the phosphoanhydride to the synthesis of a phosphodiester bond between the 5 phosphate of the incoming nucleotide and the 3 hydroxyl group of the primer.
If on the other hand ΔG is positive and you are looking up at the hill then the reaction is unfavourable or not spontaneous ie. Part C Use bond energies to estimate the enthalpy change AHrxn for Equilibrium 2. Biochemistry Questions and Answers.
Endergonic reactions absorb it. If the chemical reactions of cells were carried catalyst a substance out in a test tube most would occur too slowly to be of any use to the cell. Express your answer numerically in.
Takes in energy and is always non spontaneous. As reactions can be reversible if the forward reaction has a positive ΔG then the reverse reaction. Chemical reactions pre lab questions.
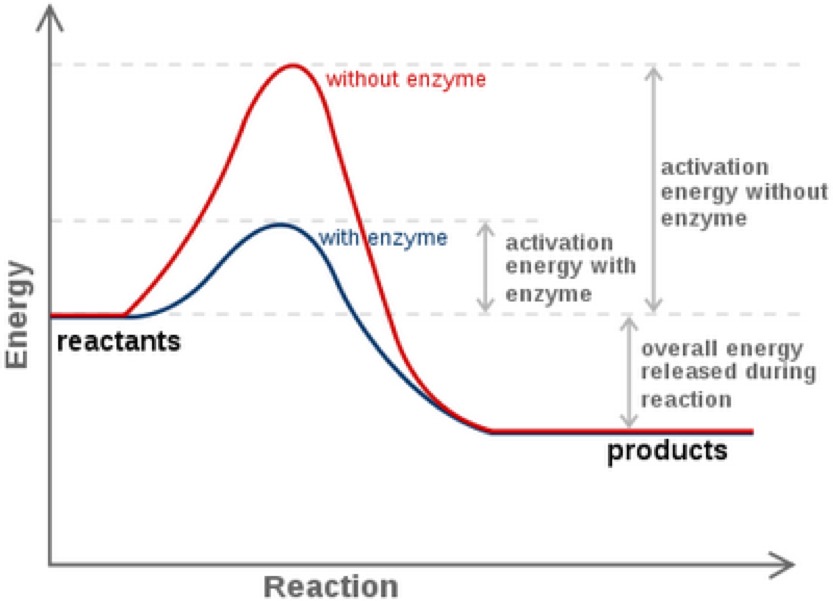
However even in an exergonic reaction a small amount of energy termed the activation energy is needed to give the reaction a kick start A good analogy is that of a match the head of which contains a mixture of energy-rich chemicals phosphorus sesquisulfide and potassium chlorate. In the less common case of endothermic reactions the situation is the reverse. This energy is referred to as the activation energy of a reaction.
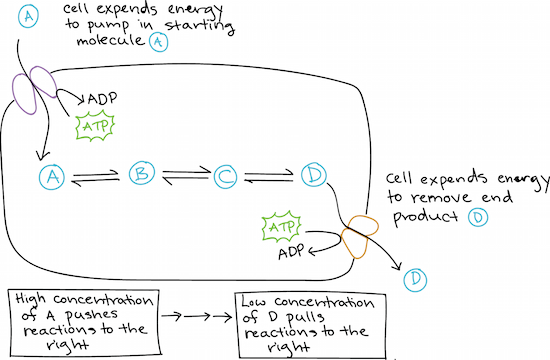
There are variety of factors that impact CO2R activity and selectivity including the catalyst surface structure morphology composition the choice of electrolyte ions and pH. Exergonic reactions release energy. For example that speeds up the suppose you performed the reaction of.
Get help with your Biochemistry homework. The potential energy of the products is less than the potential energy of the reactants. To date copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols such as ethylene and ethanol from electrochemical CO2 reduction CO2R.
However even in an exergonic reaction a small amount of energy termed the activation energy is needed to give the reaction a kick start A good analogy is that of a match the head of which contains a mixture of energy-rich chemicals phosphorus sesquisulfide and potassium chlorate. A reaction is said to be exothermic or exergonic if the final state is lower on the energy scale than the initial state. Oxidation reduction Note that adding.
When a match burns it releases substantial amounts of light and heat. Which of the following statements is true of an exergonic reaction but NOT true of an endergonic reaction. What is the difference between an exergonic and endergonic reaction.
When a match burns it releases substantial amounts of light and heat. ΔΗΧ 348 kJ Submit Previous Answers X Incorrect. If the activation energy for a reaction is large it means the reaction will take place very slowly.
5 attempts remaining Your answer is the energy change associated with breaking a carbon-carbon single bond. Chemical reactions are usually not possible unless the reactants surmount an energy barrier known as the activation energy. The reaction is ENDERGONIC or in the case of heat endothermic.
Volcanoes and volcanology Geology electron energy and light answer sheet Bing pdfdirff com Read PDF Electron Energy And Light Extension Questions Pogil Answers sustainable energy discussed in this book will be available in the long term past the remaining availability of carbon energy and is also energy that will not tip the climate into warmer conditions. In a redox reaction the loss of electrons from one substance is called _____ and the addition of electrons to another substance is known as _____. Access the answers to hundreds of Biochemistry questions that are explained in.
Physical Chemistry Thermodynamics Structure and Change 10th ed Peter Atkins Julio de Paula 2014. The speed of a chemical reaction at a given temperature T is.
Potential Kinetic Free And Activation Energy Biology 2e

Exergonic Example Chemical Reaction Process What Is An Exergonic Reaction Video Lesson Transcript Study Com

Exergonic Reactions Some Chemical Reactions Release Energy Happen Spontaneously Ex Hcl Zn H Zncl Endergonic Reactions Some Chemical Reactions Ppt Download

Structural Biochemistry Volume 1 Wikibooks Open Books For An Open World

Exergonic Reactions Some Chemical Reactions Release Energy Happen Spontaneously Ex Hcl Zn H Zncl Endergonic Reactions Some Chemical Reactions Ppt Download

Energy Matter And Enzymes Microbiology

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
Exergonic Reaction Definition Examples And Quiz Biology Dictionary

Energy Matter And Enzymes Microbiology

Exergonic Example Chemical Reaction Process What Is An Exergonic Reaction Video Lesson Transcript Study Com
Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
Soccer Science Activation Energy Dynamo Theory

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
4 1 Energy And Metabolism Concepts Of Biology 1st Canadian Edition


Comments
Post a Comment